New article by Clément Layet, Santaella, C., Auffan, M., Chevassus-Rosset, C., Montes, M., Levard, C., Ortet, P., Barakat, M., Doelsch, E. on "Phytoavailability of silver at predicted environmental concentrations: does the initial ionic or nanoparticulate form matter?" in Environmental Science Nano
Abstract
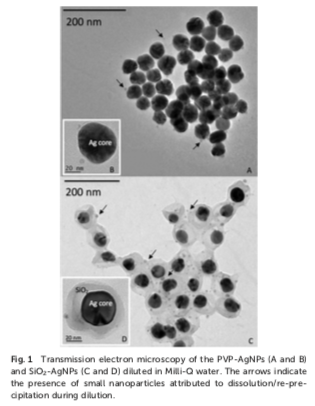
Silver phytoavailability in exposure scenarios close to predicted environmental concentrations (PEC) has rarely been studied. The ISO-standardized RHIZOtest, based on a root mat technique, was used to expose tall fescue (Festuca arundinacea) to silver at a concentration close to 0.0015 mg kg−1 and 100 times higher (0.15 mg kg−1) than PEC in soils (the PEC of Ag nanoparticulate in the soil range from 6 × 10−6 to 1.4 × 10−3mg kg−1). Silver was supplied in the form of nanoparticles with organic (PVP) or inorganic (SiO2) coating, or as dissolved Ag (AgNO3), in different soil types, with contrasting properties, including pH, cationic exchange capacity, and carbonate, organic matter, and clay contents. The Ag concentration was quantified in plant roots and leaves and in soil solutions using ICP-MS spectrometry. Multivariate analyses showed that the form of Ag, nanoparticulate or ionic, had no impact on either the flux or the translocation of Ag in plants ( p-value > 0.05). At a silver concentration close to PEC, Ag phytoavailability from Ag NPs or AgNO3 was indistinguishable from that of geogenic Ag. At 100× PEC, the type of soil, mainly clay and carbonate con- tents, controlled the Ag flux. While both decreased Ag in soil solution, clays and carbonates showed antag- onistic actions in modulating the Ag flux. We hypothesize that proton root exudation locally dissolves car- bonates and releases phytoavailable Ag, while immobilizing Ag on the edges of clays.
http://dx.doi.org/10.1039/c8en00644