A new article on the Serenade project is published.
Interaction of silver nanoparticles with metallothionein and ceruloplasmin: impact on metal substitution by Ag(I), corona formation and enzymatic activity
Liu, W., Worms, I., Herlin-Boime, N., Truffier-Boutry, D., Michaud-Soret, I., Mintz, E., Vidaud, C., Rollin-Genetet, F. 2017.
Abstract
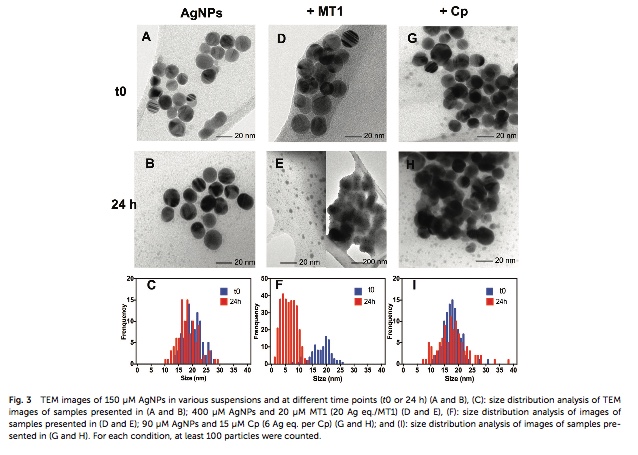
The release of Ag(I) from silver nanoparticles (AgNPs) unintentionally spread in the environment is sus- pected to impair some key biological functions. In comparison with AgNO3, in-depth investigations were carried out into the interactions between citrate-coated AgNPs (20 nm) and two metalloproteins, intra- cellular metallothionein 1 (MT1) and plasmatic ceruloplasmin (Cp), both involved in metal homeostasis. These were chosen for their physiological relevance and the diversity of their various native metals bound because of thiol groups and/or their structural differences. Transmission electron microscopy (TEM), and dynamic light scattering (DLS), UV-vis and circular dichroism (CD) spectroscopies were used to study the effects of such intricate interactions on AgNP dissolution and proteins in terms of metal exchanges and structural modifications. The isolation of the different populations formed together with on-line quantifi- cations of their metal content were performed by asymmetrical flow field-flow fractionation (AF4) linked to inductively coupled plasma mass spectrometry (ICP-MS). For the 2 proteins, Ag(I) dissolved from the AgNPs, substituted for the native metal, to different extents and with different types of dynamics for the corona formed: the MT1 rapidly surrounded the AgNPs with the transient reticulate corona thus promot- ing their dissolution associated with the metal substitution, whereas the Cp established a more stable layer around the AgNPs, with a limited substitution of Cu and a decrease in its ferroxidase activity. The accessibility and lability of the metal binding sites inside these proteins and their relative affinities for Ag(I) are discussed, taking into account the structural characteristics of the proteins.
see DOI: 10.1039/c7nr01075c